When it comes to research, few things are as important as accuracy, including accuracy in data reporting, accuracy in methodology and certainly accuracy in weighing and other measurements. Reliable, reproducible weighing results are critical for scientists. In this blog, we’ll take a look at the critical role the accuracy of lab balances plays in scientific research.
What is Accuracy and Why is it Important?
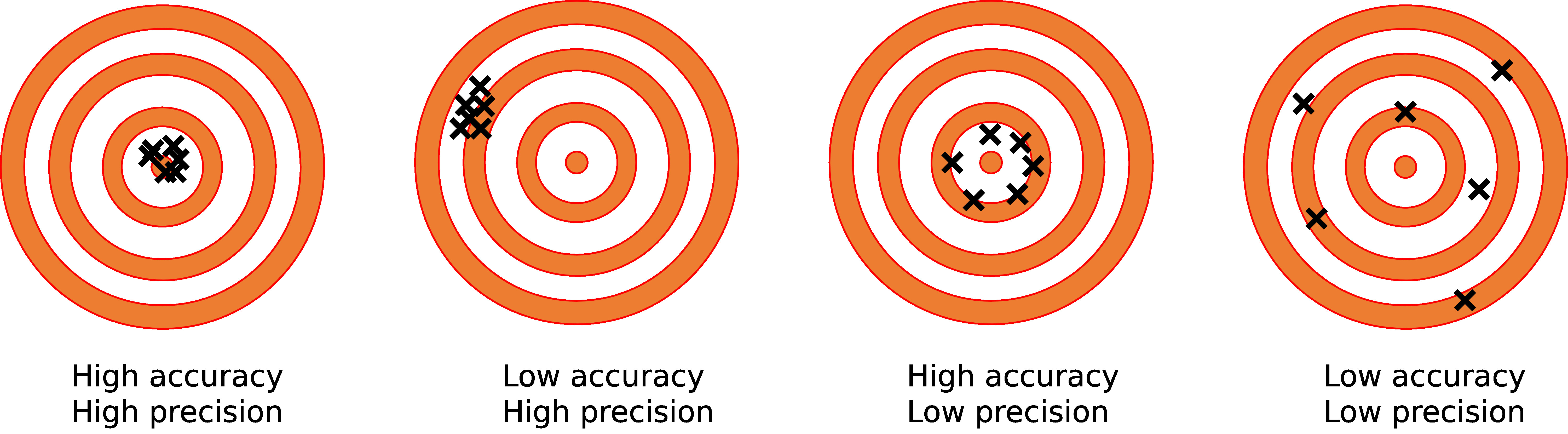
First, let’s define accuracy as it applies to weighing scales and balances. In our glossary, we define it as the “ability to display a value that matches the ideal value for a known weight.” Put more simply, it’s how close the measured value is to the actual value.
Though they’re related, accuracy and precision are sometimes confused, despite being quite different. Your analytical balance, for example, can be precise to a degree of 0.0001g, but if the reading isn’t accurate (perhaps the balance needs to be calibrated) then the precision won’t matter.
If the weighing result contains even minor errors, the validity of an entire experiment can be called into question at best, or completely invalidated at worst. Experiments are performed by multiple scientists and if errors creep in, that can impact the reproducibility of the results, as well as resulting in loss of time and money (reagents can be expensive).
How Do You Know Your Balance is Accurate?
To be sure your balance is accurate, you need to perform a calibration and verification. The process involves comparing weighing results from your device with the weights of a calibration weight to see how closely they match.
There are two kinds of calibration (external calibration, which uses calibration weights purchased separately, and internal calibration, which features calibration weights built into the weighing device for calibration at the touch of a button) and Adam Equipment offers balances with both options, as well as calibration weights for users to perform an external calibration.
(1) Purchase calibration weights with known, traceable values. Your weights should cover the range of measurements that your balance is designed to handle.
(2) Make sure your balance is clean, free of debris and level.
(3) Zero out your balance, then weigh a standard calibration weight several time and record the results.
(4) Determine the average reading by adding your results and dividing by the number of readings.
(5) Compare the average to the standard weight. The closer your average is to the standard reading, the more accurate your balance.
(6) To determine your balance’s percentage of accuracy, use the formula Accuracy (%) = (Measured Value / Standard Value) * 100. For example, if the average reading from your balance for a standard weight of 100 grams is 99 grams, the accuracy would be Accuracy (%) = (99 / 100) * 100 = 99%
(7) Compare your balance’s accuracy to industry standards for your application.
(8) If the accuracy isn’t acceptable for your needs, the balance may need to be calibrated or adjusted. Consult your user manual or the balance’s manufacturer for guidance.
(9) Repeat the process at regular intervals. Repeat more often if your device is subject to heavy use or environmental factors that may affect its accuracy.
Accuracy Vs. Standard Deviation
While accuracy and standard deviation are two distinct concepts, they’re very much tied together. While accuracy is defined as how close a balance’s weighing result is to the actual value, standard deviation provides the range of measurements when weighing the mean (actual) value.
A low standard deviation means that the measurements are very close to the mean value, providing higher precision. A high standard deviation indicates a greater variability in the weighing results, which can result in lower precision.
In our blog Why Balance Testing is Important, we discuss how to calculate your balance’s standard deviation.
How are Lab Balances Used in Research?
A wide variety of scientific disciplines – including chemistry, biology, physics, and pharmaceuticals – rely on lab balances during a wide range of tasks and applications:
- Sample preparation, such as weighing reagents, compounds and solutions
- Analytical techniques like titrations and chromatography require precise weighing results to determine sample concentrations or purity
- Formulation & Quality Control for pharmaceutical research, ensuring accurate dosing and compliance with regulatory standards
- Environmental studies, including weigh water and soil samples to determine ecosystem health and monitor pollutants (see our blog on "How Laboratory Balances are Used in Environmental Sciences")

Barriers to Weighing Accuracy in Labs
What kinds of issues can impact the accuracy of weighing results in labs? Calibration, for one, as we discussed above. Regular calibration with certified calibration weights helps to ensure the accuracy of the results.
Semi-micro and analytical balances are extremely sensitive to conditions like vibrations, temperature, humidity and air currents. Many , and models are equipped with a draft shield to prevent air currents from affecting readings.
Adam also offers the for semi-micro and analytical balances which provides a solid, stable surface for decreasing interference from such seemingly-slight vibrations such as people walking on the floor nearby or a building’s HVAC system.

To help ensure continued accuracy of weighing results, read our blog post, "Why Balance Testing is Important."
Questions about accuracy or need assistance in choosing the right lab balance? Reach out to us with your questions and we’ll be happy to help!