This guide is designed to walk users through manual density calculations. This is how to calculate density if your balance does not have a density calculation function and a density kit. While this method is more complex, it is more precise and suitable for smaller objects.
Density is usually expressed in grams per cubic centimeters (g / m3).
You will need the following materials:
- Weighing Balance or Scale (1 place or better preferably)
- Graduated Beaker or Cylinder
- Thermometer
- Clamp to affix the thermometer to the beaker
- Object whose density needs to be measured
- Writing implements (digital or analog)
- Water or other known liquid (room temperature)
- Calculator (or more paper to do the manual calculations)
- For floating object’s density: glass plunger of known mass, volume and density
- Suspension method
- Table of densities for the water or liquid used
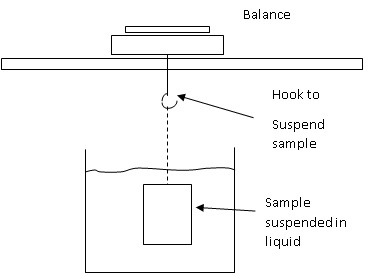
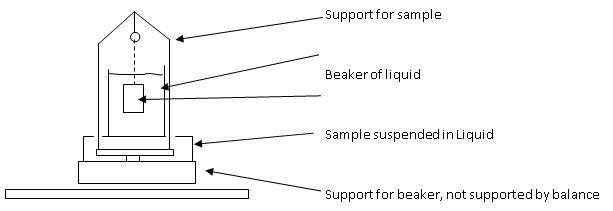
This method relies on the sample being suspended. If your balance offers an underhook, use that hook to suspend the sample. If not, you will have to set up a suspension method on top of the balance.
|
Density Set Up Under Balance |
Density Set Up On Balance |
- Make sure the scale or balance is calibrated and shows zero.
- If the balance has multiple units, set the unit to grams. If the balance does not offer grams, you must convert the result in grams.
- Set up your suspension method, on or under the balance.
- Add your liquid to the beaker. Your liquid must have a stable temperature.
- Record the liquid’s temperature. Its density will depend on it. You can find density tables for all sorts of liquids online. If you are unable to access the internet, print out a table with your liquid’s densities by temperatures beforehand.
- Tare the mass of the suspension kit. If you cannot tare, record the equipment’s mass to subtract it later.
- Suspend the object in the air. Make sure the balance is giving stable readings and only record the results when the object is motionless.
- Weigh the object in the air and record the mass in grams. This is Mair in our equation.
- Suspend the object in the liquid and record the mass once stable. Make sure there are no air bubbles and that the sample is fully submerged. This is Mliq in our equation.
- You can now calculate the density of the sample. Divide the liquid’s density by its mass in the air divided by its mass in the air minus its mass in the liquid.
| Density of Sample= Density of Liquid x | Mair |
| Mair - Mliq |